Calem Blogs
Steps to Implement CAPA in Calem
CAPA is added in Calem in the coming release of R2024b (June 2024). This blog present the steps to implement CAPA in Calem.
1. What is CAPA
The FDA (U.S. Food and Drug Administration) mandates CAPA processes for medical device manufacturers under 21 CFR 820. Here is the CAPA FDA web site. CAPA has a general appeal for an organization to get better by addressing root causes of problems, and eliminating or reducing the recurrences of problems addressed.
CAPA stands for Corrective and Preventive Action. It is a key concept in quality management systems (QMS) across various industries, particularly in manufacturing, pharmaceuticals, medical devices, and biotechnology. The purpose of CAPA is to improve processes, eliminate nonconformities, and prevent their recurrence.
by ChatGPT
Purpose/Importance
from FDA Web Site
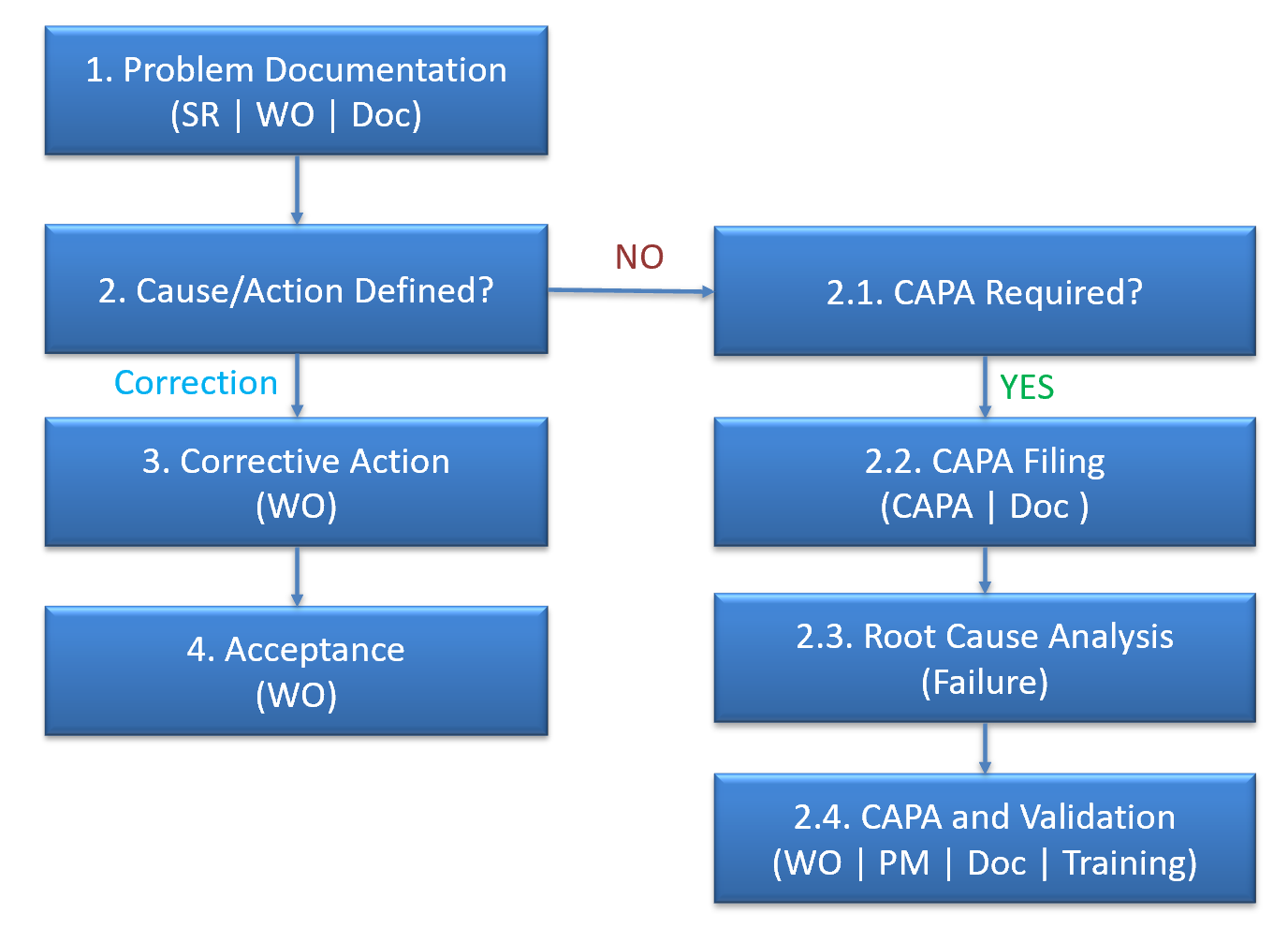
The purpose of the corrective and preventive action subsystem is to collect information, analyze information, identify and investigate product and quality problems, and take appropriate and effective corrective and/or preventive action to prevent their recurrence. Verifying or validating corrective and preventive actions, communicating corrective and preventive action activities to responsible people, providing relevant information for management review, and documenting these activities are essential in dealing effectively with product and quality problems, preventing their recurrence, and preventing or minimizing device failures. One of the most important quality system elements is the corrective and preventive action subsystem.
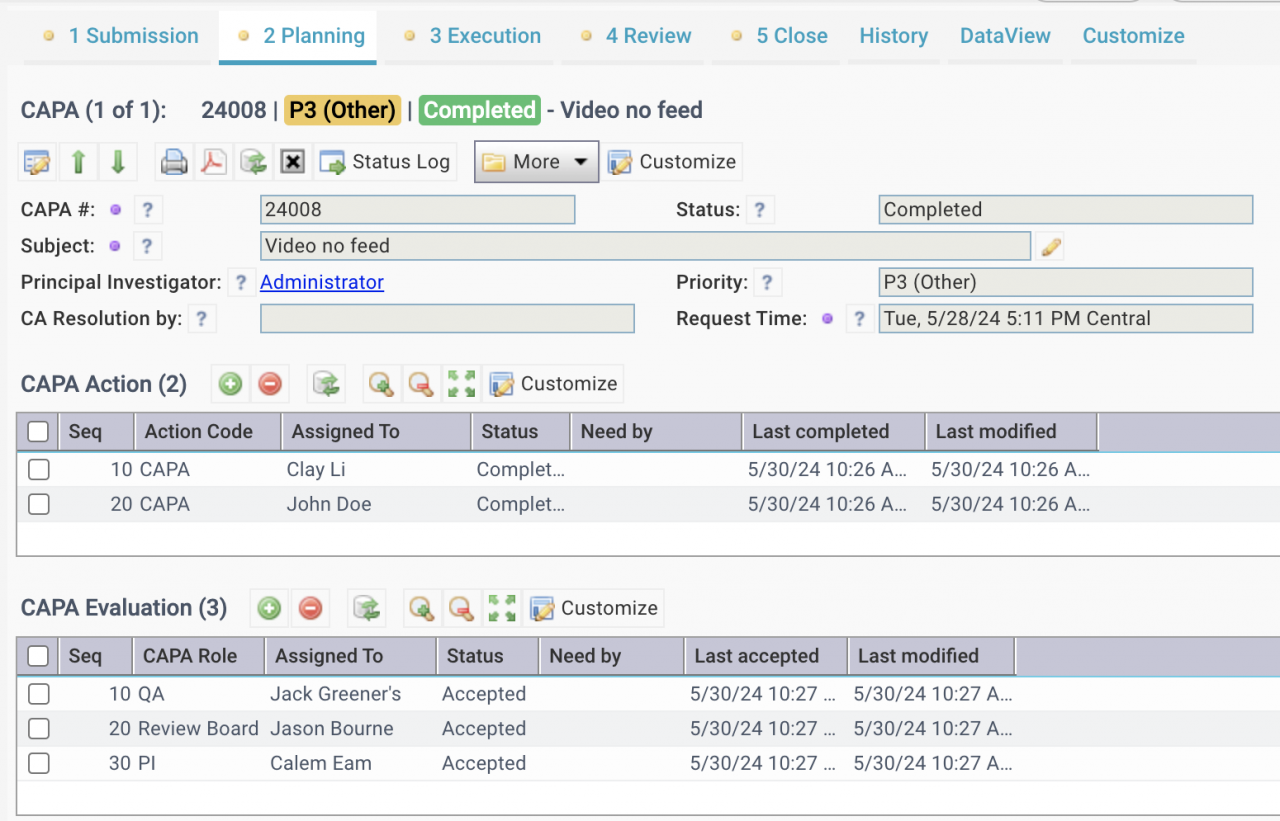
2. CAPA in Calem
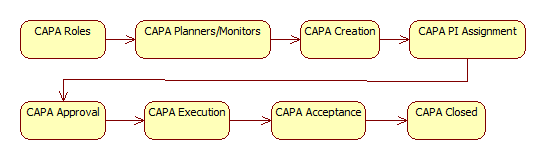
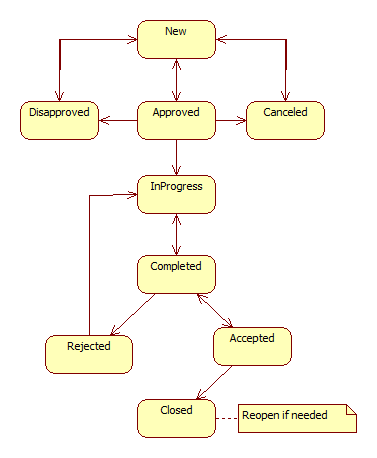
CAPA is a new module in Calem R2024b. It is integrated with Work Order and Service Request modules. Here is the flow diagram of CAPA in Calem.
3. CAPA Roles in Calem
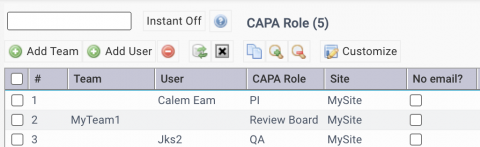
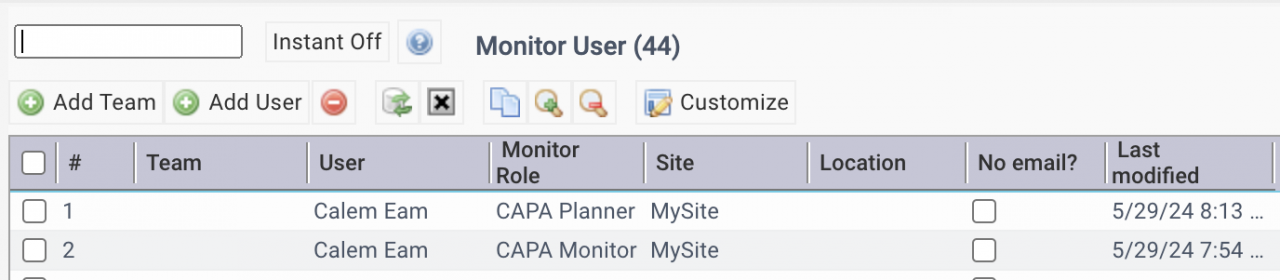
The first step of CAPA implementation is to set up the roles for CAPA.
- CAPA roles: Organization | ACL Profiles | CAPA Role
- CAPA planners and monitors: Organization | ACL Profiles | Monitor User
| Role | Note |
| CAPA Planner | Review CAPA cases and assign Principal Investigators (PIs) |
| CAPA Monitor | Receive CAPA event emails |
| PI (Principal Investigator) | Responsible for a CAPA case |
| CAPA Staff | Analyze cause and develop actions for CAPA |
| Process Staff | Responsible for process documentation for the CAPA |
| Training Staff | Responsible for the training development for the CAPA |
| Evaluation Staff | Evaluate the effectiveness of the CAPA solution |
| Requester | A user who creates a CAPA. Any users with access to the CAPA module can initiate a CAPA case. |
4. Assign Roles to CAPA
There are two types of resources to assign to a CAPA:
- The resources to work on CAPA actions. They include users assigned to a site.
- The resources to evaluate the effectiveness of the CAPA actions.
- The resources are assigned to a CAPA by a PI of a CAPA.
- These users along with the requester and CAPA planners can modify a CAPA.
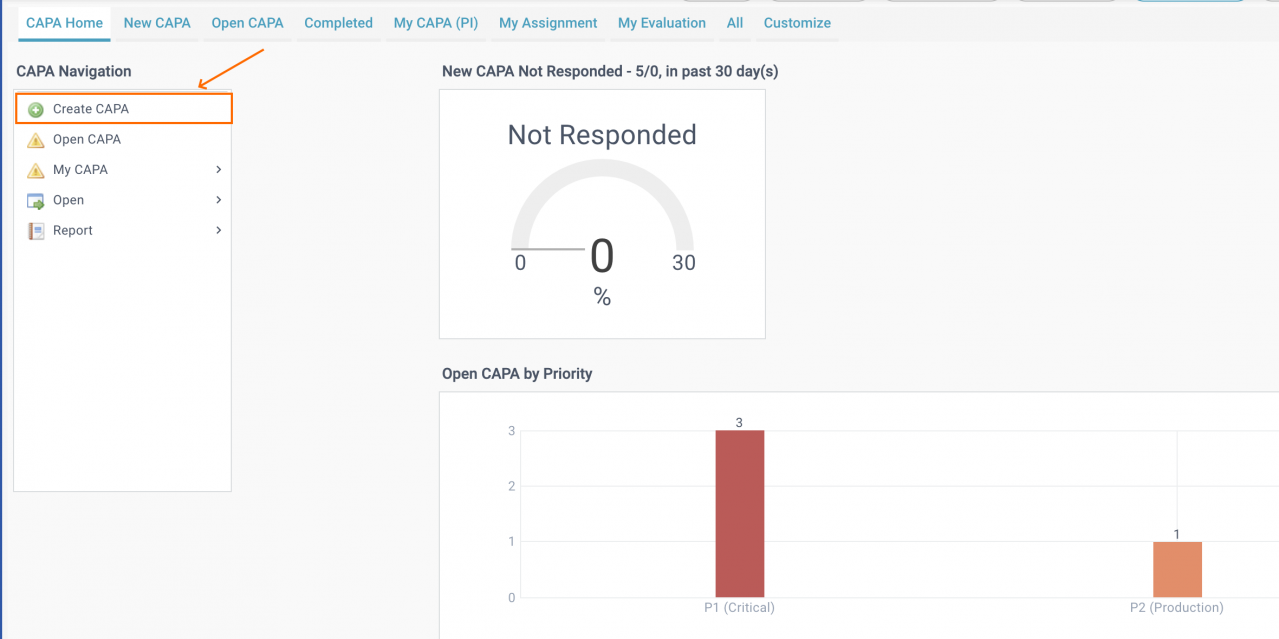
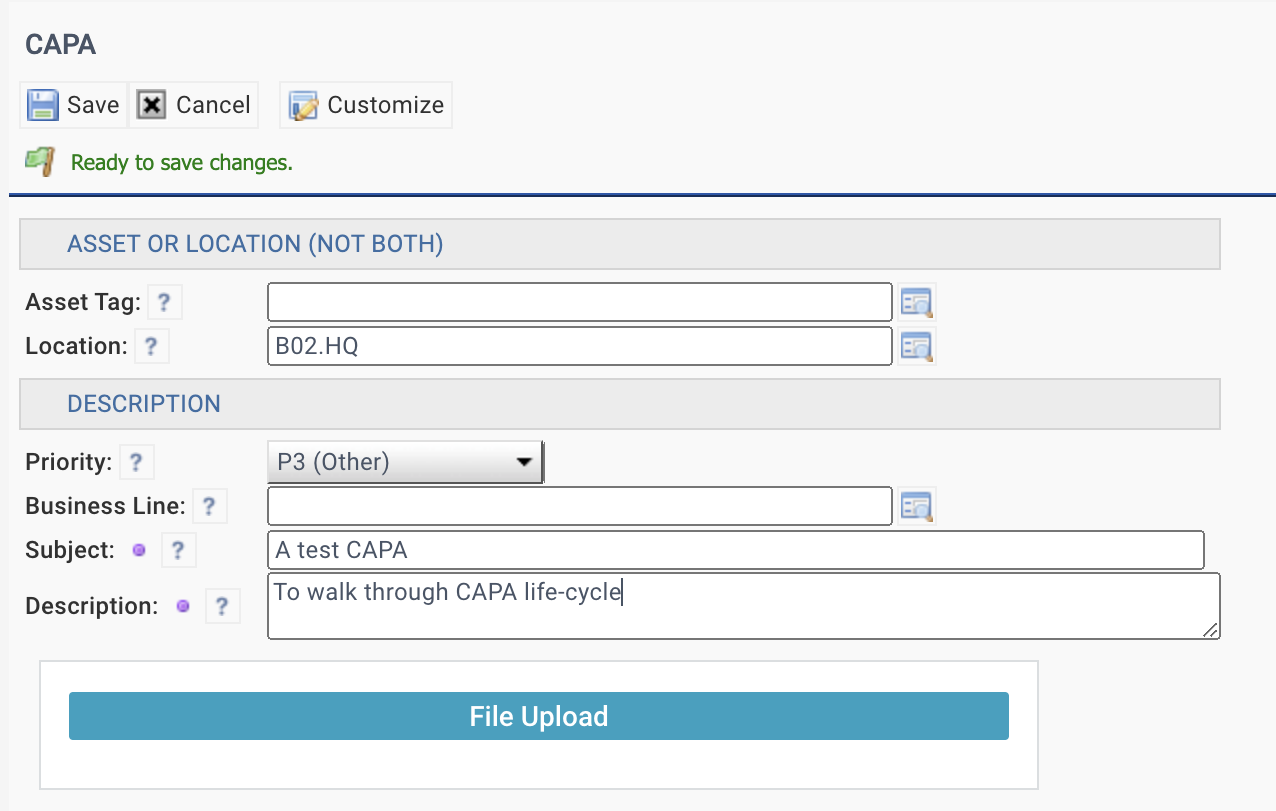
5. Create a CAPA
A CAPA case can be created by a user with access to CAPA module.
- An Asset or Location is required to create a CAPA.
- Files may be attached when creating a CAPA.
- After a CAPA is created, a PI (Principal Investigator) needs to be assigned by a CAPA planner for a site.
- The PI can build a CAPA team by assigning users for CAPA Action and Evaluation.
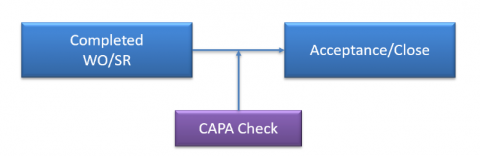
6. CAPA Integration with WO and SR
CAPA integration with WO and SR may be enabled by site or company settings.
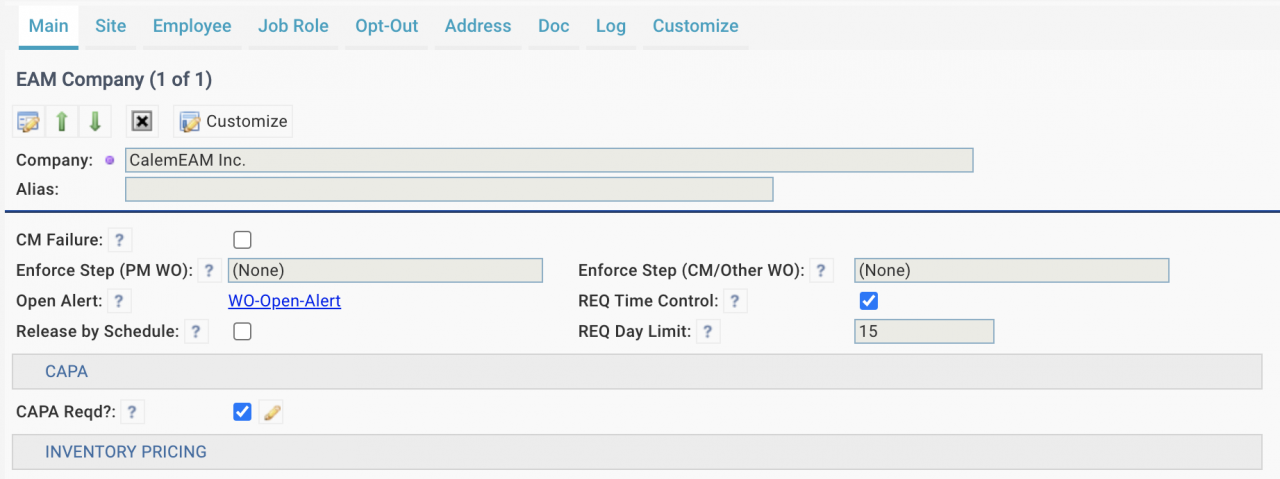
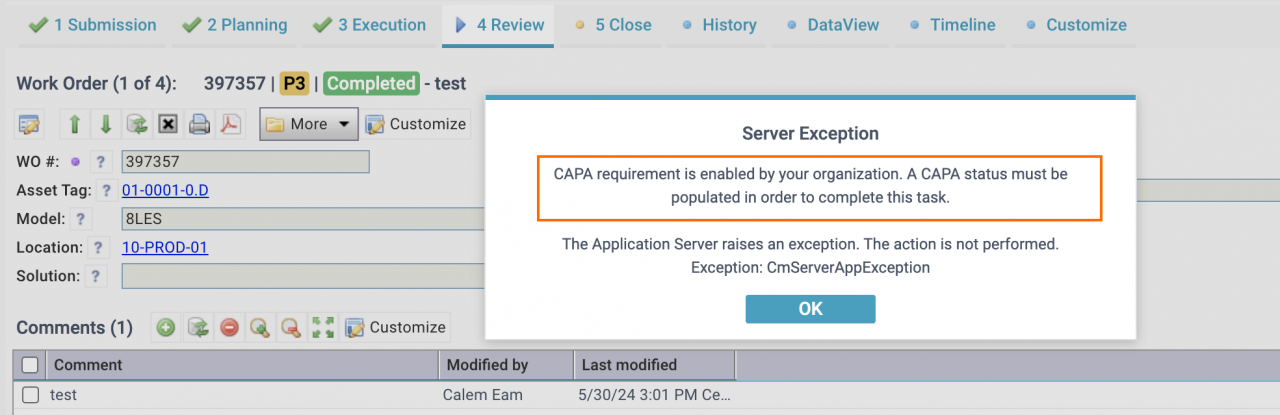
- "CAPA Reqd" may be checked to mandate CAPA status population when completing a corrective work order or a service request.
- When a corrective WO or an SR is accepted or closed, Calem will check the CAPA status of the WO or SR, If the CAPA status is not set, Calem will raise an error to block the operation.
6.1 Create WO from CAPA
WO creation is allowed for a CAPA by its PIs, planner, action users and reviewers. Work orders can be created from CAPA form: 1) Evaluation tab of TaskView; 2) Evaluation tab of DataView
7. CAPA Lifecycles
Only a Principal Investigator (PI for a CAPA) can modify CAPA statuses.
- Additional PIs can be added to a CAPA as evaluation member with the PI role.
- A CAPA action can be completed by the user assigned to it or a PI.
- A CAPA evaluation can be completed by the user assigned to it or a PI.
- A CAPA cannot be completed if there are unfinished actions.
- A CAPA cannot be accepted if there are unfinished evaluation tasks.
Additional Resources
Related Posts
By accepting you will be accessing a service provided by a third-party external to https://eam.calemeam.com/