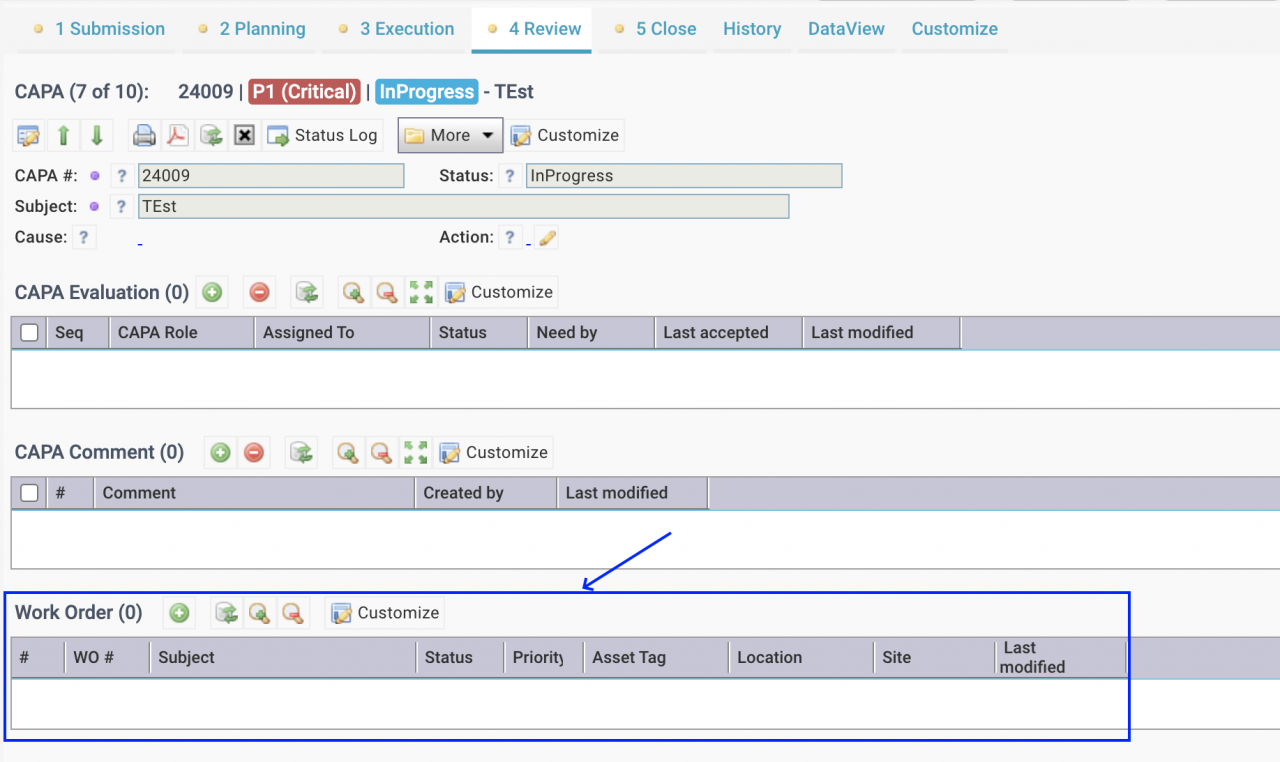
Work orders can be created for a CAPA case from the Evaluation tab of the CAPA form. Permissions to create a WO are granted to users with roles to manage a CAPA including: Principal Investigators (PIs), CAPA Planners, and users assign to action and evaluation roles.The work order creation screen is launched with asset and location of the ...
684 Hits
684 Hits